Lead the Way in Design with KINETIQX
The ultimate plug-n-play prototyping portal

Key Benefits of KINETIQX
Developed under design controls, ISO/IEC compliant.
Unlock More Value with Connected Prototyping
KINETIQX provides a complete, plug-n-play prototyping portal that empowers you to create connected medical prototypes quickly and efficiently. The platform is engineered to increase the likelihood of regulatory approval and accelerate time-to-market, so you can bring new products to market faster.
With a streamlined design approach, you gain access to a pre-built, validated system that minimizes initial expenditures and maximizes your development efficiency.

Revolutionize Your Prototyping Process
Transform your prototyping toolbox with KINETIQX and start creating connected prototypes, enhancing your value proposition. Gain the ability to develop cutting-edge, FDA-approved, and CE-certified connected medical solutions, enabling a seamless progression from prototype to production.
Built under a rigorous Quality Management System, KINETIQX guarantees that every prototype meets industry standards, enhances efficiency, and accelerates development timelines, delivering unmatched value to your MedTech clients.
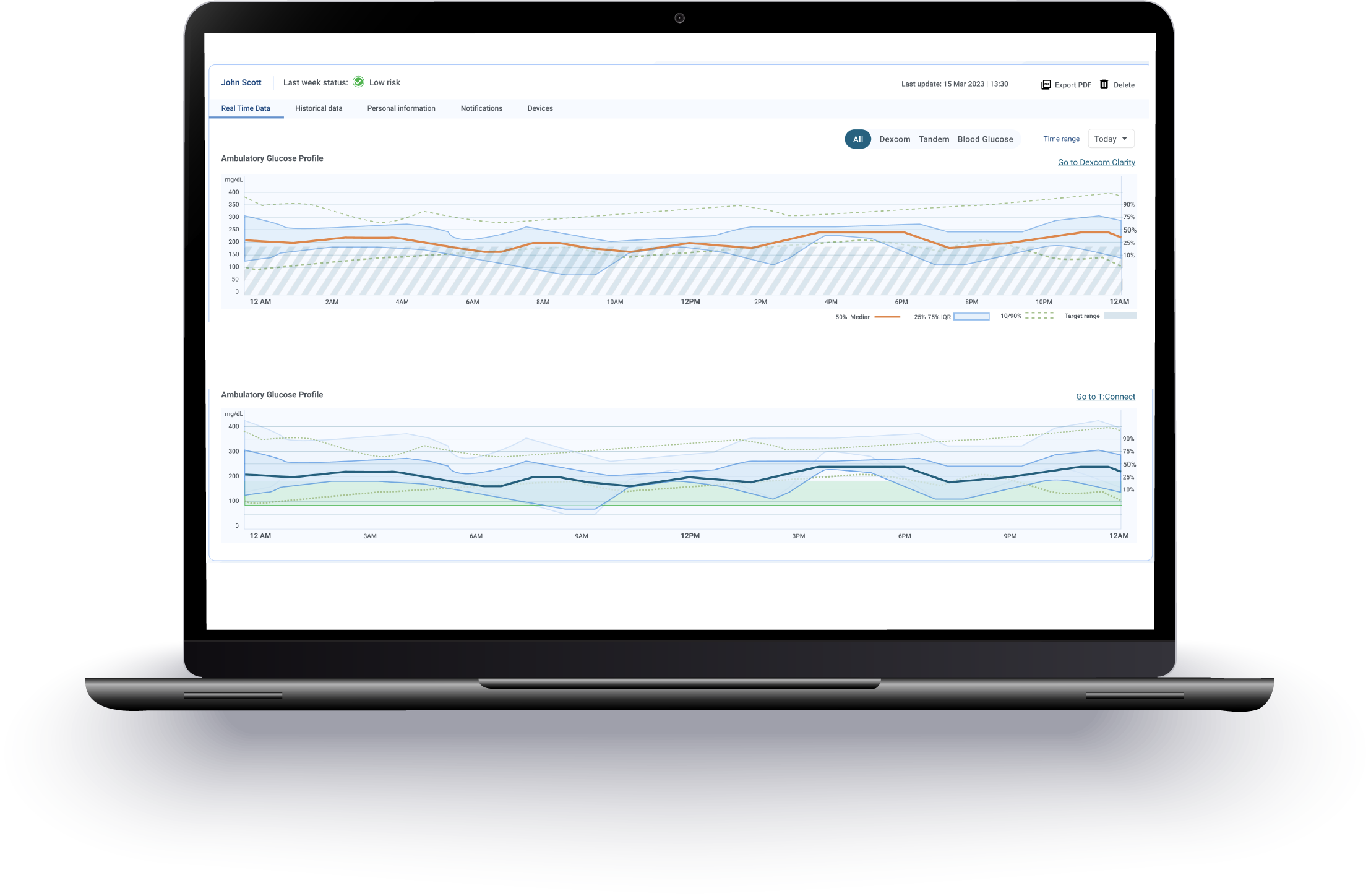
Stay ahead of industry trends by leveraging real-time data generated during prototyping, and make informed, data-driven decisions that elevate your connected designs.
Elevate Your Development Game
Boost your revenue, outpace the competition, and set the stage for successful market launches with KINETIQX.

